Introduction
The Hygroscopic Cycle was created and developed by Francisco Javier Rubio Serrano in 2008 and is located in the state of the art since 2010, as "Rankine Cycle with absorption step by hygroscopic compounds".
In 2012 IMASA, Engineering and Projects, S.A. (www.imasa.com), acquired the rights to take advantage of the technology. The company's innovation IBERGY (www.ibergy.com) led by Rubio himself with José Luis Barrientos Reguera and Carlos Pérez Rivero, is developing all the chemical salt studies and the equipment for the benefit of the cycle.
It is a power cycle working with hygroscopic compounds which optimize the output steam of a condensing turbine, and can work under high vacuum at the output thereof and good refrigeration conditions. In short, the condensation temperature at a given pressure in the condenser is increased.
The Hygroscopic Cycle is a power cycle working with water and hygroscopic compounds, which must have the following features:
- They have to be hygroscopic compounds, deliquescent materials.
- Must be less volatile than water and easily separable, that retention may be reversible and the steam can be easily desorbed.
- Must be chemically stable at the pressures and temperatures of steam cycle work to which it will be subjected.
- No toxic or flammable fluids are advised.
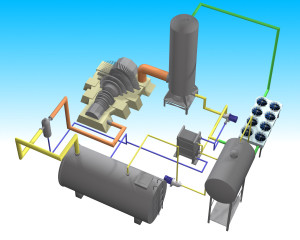
The Hygroscopic Cycle incorporates physical and chemical principles of absorption machines with the aim to provide to Rankine cycle higher performance and better cooling. It includes the following main equipment:
- Steam turbine.
- Steam absorber.
- Condensate pump.
- Solution pump.
- Enthalpy recuperator.
- Deaerator.
- Steam generator.
- Superheater.
- Air cooler.
This cycle may include all improvements incorporated into the Rankine cycle (pressure increase start of expansion, the pressure decrease end expansion vapor superheat, reheat, regeneration, supercritical conditions).
Background
The Rankine cycle is a power cycle which operates with steam. It is currently used in the generation of electricity thermoelectric plants. Water is used as working fluid passing through different equipment and producing a mechanical work through its expansion in the inside of a steam turbine, a generator connected to the mechanical shaft of the turbine produces the electrical output stream.
The performance of the Rankine cycle is determined by dividing the remainder of the work produced by the turbine and that consumed by the pump, divided by the heat of the steam transferred from the steam generator.
Numerous improvements have been introduced in Rankine cycle over the years with the aim of increasing the efficiency of the cycle, and thereby improve the efficiency of the power plant. In addition to increasing the performance of the equipment that take part in the process (pump, turbine, boiler...), it has become necessary to alter the layout of the plant. The main improvements to increase performance of the Rankine cycle are the following:
- Superheating of steam at the beginning of expansion (Hirn cycle).
- Modifications of the operating conditions at the beginning and the end of expansion, increase of the initial pressure of expansion, decresase of pressure at the end of expansion, supercritical conditions.
- Reheating (working with higher pressures, avoiding the formation of moisture at the end of the expansion).
- Regeneration (the feed water is preheated before it reaches the boiler using open or closed heaters with the steam of the extractions of steam turbine).
- Binary cycle (two circuits in serial, one high temperature working and the other one a low temperature).
- Decreasing the pressure of the expansion term. This is limited by the temperature of the coolant (water or air mainly) in the condenser.
Hygroscopic compounds
Hygroscopic compounds are all those substances that attract water in vapor or liquid from its environment, thus its main application is as desiccants. Many react chemically with water such as metal hydrides or alkali metals. Others take as water of hydration in its crystal structure such as sodium sulfate. Water may also be physically adsorbed. In the latter two cases, retention is in a reversible mode and water can be desorbed. In the first case, it can not be recovery in a simple way.

Hygroscopic salts
The deliquescent materials are substances (in its most salts) that have a strong chemical affinity for moisture and absorb relatively high amounts of water if exposed to the atmosphere, forming a liquid. We have examples of deliquescent such as calcium chloride, ferric chloride, magnesium chloride, zinc chloride, potassium carbonate, potassium hydroxide and sodium hydroxide. The presence of these compounds in dilution with water modifies the properties of the same in relation to its pure state. These modifications are referred to as properties of a solution, constituent (viscosity, density, electrical conductivity, etc.) and colligative (decrease of the solvent vapor pressure, boiling point elevation, freezing point lowering and pressure osmotic) of special interest in this technology.
One of the main applications of the hygroscopic compounds is absorption cycles used for refrigeration. These machines began to commercialize in the early 50´s, though his principle has been known for over a hundred years. The absorption cycles are physically based on the ability of some substances, such as water and certain salts, to absorb, in liquid phase, vapors of other substances such as ammonia and water, respectively. Similarity in this cycle water is the refrigerant and the hygroscopic compound the absorbent.
Examples of several known hygroscopic compounds are:
- Sodium chloride (halite) (NaCl).
- Calcium chloride (CaCl2).
- Sodium hydroxide (NaOH).
- Sulfuric acid (H2SO4).
- Copper sulphate (CuSO4).
- Phosphorus pentoxide (P2O5 or more correctly P4O10).
- Silica gel.
- Hydrated salts such as Na2SO4∙10H2O.
- LiBr (the most widely used at present, especially in absorption machines for cold generation).
- LiCl.
- Amines.
Publications
The Hygroscopic Cycle in the media:
> Continue to Process.